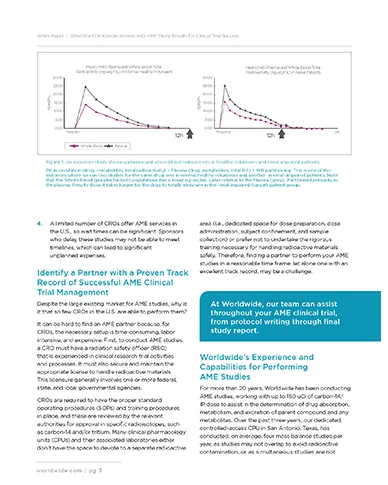
While AME studies traditionally have been conducted during Phase II clinical development or later, earlier investment in these activities — on the heels of preliminary studies, such as first-in-human, dose escalation, and food effect — often proves worthwhile. Furthermore, the FDA has recently requested AME data sooner in the development process. Proactive AME studies will help lay the groundwork for the advanced phases of your research and provide valuable data to satisfy regulators as needed.
Download our white paper to learn more about:
- Key factors that impact your partnership decisions
- Best practices in clinical design for optimal trial outcomes
- Operational factors for effectively running an early AME study in your new chemical entity